1. Introduction
The Earth is surrounded by several protective atmospheric layers that make life possible. Among them, the Ozone Layer plays a highly crucial role in shielding all forms of life from intense solar ultraviolet radiation. Without this layer, harmful UV rays would penetrate the Earth’s surface, causing severe damage to humans, animals, plants, and entire ecosystems. For over a century, industrial activities have introduced chemicals into the atmosphere that directly damage the Ozone Layer, resulting in what is known as Ozone Depletion. Scientists, governments, and environmental organizations continue to work together to reverse the harm and protect the planet’s future.
Through scientific research and international cooperation, major progress has been made, but continuous awareness and action are still necessary to ensure long-term safety. This article provides a comprehensive and research-based explanation of the Ozone Layer, its functioning, importance, location, causes of depletion, its harmful effects, global solutions, recovery predictions, and the role that individuals and societies can play.
2. What is the Ozone Layer?
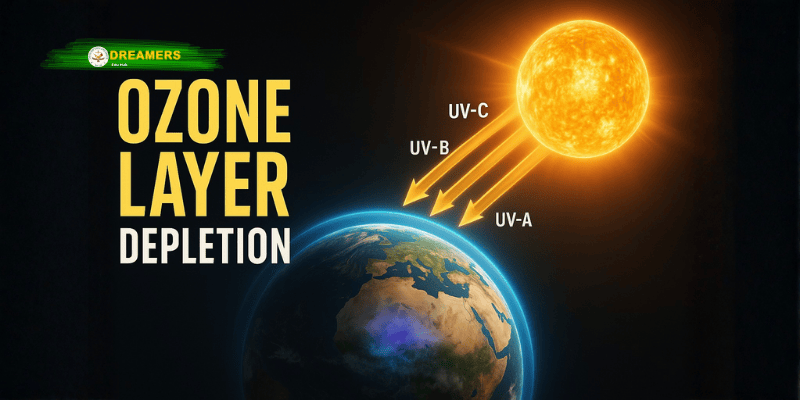
The Ozone Layer is a region in the Earth’s stratosphere that contains a higher concentration of ozone (O₃) molecules compared to other parts of the atmosphere. Ozone is a form of oxygen made up of three oxygen atoms bonded together. While oxygen (O₂) supports breathing and combustion processes, ozone (O₃) acts primarily as a protective barrier.
The Ozone Layer absorbs and blocks around 97–99% of the Sun’s harmful ultraviolet radiation, especially UV-B and UV-C rays. These rays can damage DNA and cellular structures in living organisms. The presence of this layer has allowed complex forms of life to evolve and survive on Earth.
3. How Does the Ozone Layer Work?
The functioning of the Ozone Layer depends on a natural chemical reaction involving sunlight and oxygen. The Sun continuously emits ultraviolet rays. When these rays reach the stratosphere:
-
UV-C rays break down molecular oxygen (O₂) into single oxygen atoms (O).
-
These free oxygen atoms react with O₂ molecules to form ozone (O₃).
-
Ozone absorbs UV radiation and breaks back into O₂ and O atoms.
-
The cycle repeats continuously.
This natural balance is known as the Ozone-Oxygen Cycle.
When industrial chemicals release chlorine or bromine atoms into the atmosphere, they interrupt this balance. The result is reduced ozone concentration, leading to Ozone Depletion.
4. Where is the Ozone Layer Located?
The Ozone Layer is found in the Stratosphere, about 10–50 kilometers above Earth’s surface. The maximum concentration of ozone exists at altitudes between 20–30 kilometers.
Layers of the atmosphere from bottom to top:
• Troposphere (0–10 km): Where humans live and weather occurs
• Stratosphere (10–50 km): Ozone Layer location
• Mesosphere (50–85 km)
• Thermosphere (85–600 km)
• Exosphere (outer boundary)
The stratosphere contains the ideal conditions (oxygen, sunlight, and low air density) needed for ozone formation.
5. Formation of the Ozone Layer
The Chapman Cycle explains how ozone is formed:
• Step 1: High-energy UV radiation splits O₂ into single oxygen atoms
• Step 2: Single atoms attach to O₂ molecules to form O₃
• Step 3: UV energy continues to break O₃ into O₂ + O
• Step 4: Reactions repeat constantly
Natural nitrogen and hydrogen compounds regulate how fast ozone forms and breaks apart, maintaining a stable equilibrium. The problem arises when additional destructive chemicals are introduced by humans.
6. Why is the Ozone Layer Being Damaged?
The major cause of Ozone Depletion is the release of Ozone-Depleting Substances (ODS). These chemicals rise slowly into the stratosphere, where UV radiation breaks them down, releasing chlorine and bromine atoms that destroy ozone molecules.
One chlorine atom can destroy over 100,000 ozone molecules, making even small concentrations extremely harmful.
Sources of harmful ODS:
• Refrigeration and Air Conditioning (older systems)
• Aerosol sprays and propellants
• Industrial solvents
• Fire extinguishers (halons)
• Chemical manufacturing
• Agricultural pesticides
Even after their usage is stopped, these chemicals remain in the atmosphere for decades.
7. What is the Ozone Hole?
The term Ozone Hole refers to the severe reduction in ozone concentration over a specific region, observed primarily above Antarctica. It is not an actual hole but a region where the thinning of the Ozone Layer becomes unusually extreme. The Ozone Hole was first discovered in the mid-1980s by British scientists from the British Antarctic Survey, who used both ground-based instruments and satellite data to confirm that ozone levels had dropped dramatically. Later, NASA satellites verified this alarming depletion pattern in the stratosphere.
This thinning becomes worse during the Antarctic spring months (September to November) when returning sunlight accelerates ozone destruction. During the long winter in Antarctica, extremely cold temperatures lead to the formation of Polar Stratospheric Clouds (PSCs). These clouds provide a chemical surface on which chlorine and bromine compounds—released from man-made chemicals like CFCs—are converted into their reactive and ozone-destroying forms.
When sunlight returns in early spring, these activated chlorine and bromine atoms rapidly break down large numbers of ozone molecules, creating the seasonal Ozone Hole. Due to the persistent polar vortex, which traps air over Antarctica, the damaged ozone cannot be replaced easily by ozone-rich air from other regions, making the hole more intense and long-lasting. Scientific monitoring has shown that although international actions have slowed the depletion, the Ozone Hole still appears every year and remains one of the strongest indicators of human impact on the atmosphere.
8. Why Does the Ozone Hole Mainly Occur Over Antarctica?
The Ozone Hole is most prominent over Antarctica due to:
• Extremely low temperatures during winter
• Formation of Polar Stratospheric Clouds (PSCs)
• Trapped circulation winds (polar vortex)
• Rapid ozone destruction when sunlight returns
These unique environmental conditions make the region more vulnerable to chlorine-driven reactions.
9. Chemicals Responsible for Ozone Depletion
Major Ozone-Depleting Substances (ODS):
• CFCs: Chlorofluorocarbons used in refrigerators and air conditioners
• HCFCs: Hydrochlorofluorocarbons, later replacements but still damaging
• Halons: Fire suppression systems
• Carbon tetrachloride: Industrial solvents
• Methyl chloroform: Cleaning agents
• Methyl bromide: Agricultural pesticide
• Nitrous oxide (N₂O): Now the largest ozone-depleting emission from human activities
These chemicals have long atmospheric lifetimes, making recovery slow.
10. Effects of Ozone Layer Depletion
Impact on Human Health
• Increased skin cancer cases (melanoma and non-melanoma)
• Eye damage and increased cataract rates
• Weakened immune response
• Faster skin aging and sunburn incidents
Impact on Animals
• Eye and skin disorders in terrestrial animals
• DNA damage and weakened reproductive systems
• Reduced survival rates in wildlife
Impact on Marine Ecosystems
• Devastating impact on phytoplankton, the base of marine food chains
• Decline in fish populations
• Reduced ocean biodiversity
• Damage to coral reefs from UV-induced stress
Impact on Plants and Agriculture
• Reduced crop yield and quality
• Interrupted photosynthesis
• Damage to leaf tissues
• Threat to food security in vulnerable regions
Environmental and Climate Damage
• Greater heat absorption leading to additional warming
• Disruption of climatic balance
• Interaction with greenhouse gases worsens climate change
11. Difference Between Ozone Depletion and Global Warming
Many people confuse Ozone Depletion with global warming, but they are different issues.
| Factor | Ozone Depletion | Global Warming |
|---|---|---|
| Main Cause | Chlorine and bromine chemicals like CFCs | Greenhouse gases like CO₂ |
| Effect | Thinning of the Ozone Layer | Rise in global temperatures |
| Result | More UV radiation reaches Earth | Melting glaciers, sea-level rise |
| Human Risk | Skin cancer, cataracts | Floods, heatwaves, storms |
However, some chemicals like CFCs contribute to both problems simultaneously.
12. Global Actions to Protect the Ozone Layer
This environmental issue has prompted worldwide cooperation.
Major international agreements:
Vienna Convention (1985)
• Laid the foundation for global ozone protection policies
Montreal Protocol (1987)
• Banned and phased out Ozone-Depleting Substances globally
• Recognized as the most successful environmental treaty in history
• Supported research and replacement with safer chemicals
Kigali Amendment (2016)
• Targeted HFCs, preventing future climate change impacts
Due to strong cooperation, atmospheric concentrations of CFCs have dropped significantly.
13. Technological Alternatives to ODS
Industries have shifted to eco-friendly technologies:
• Hydrofluoroolefins (HFOs) for refrigeration
• Water-based and natural refrigerants like ammonia and CO₂
• Advanced fire suppression agents without halons
• Safer solvent systems for industries
Such innovations allow sustainable development without compromising environmental safety.
14. Can the Ozone Layer Recover Fully?
According to the United Nations Scientific Assessment:
• Global Ozone Layer is expected to recover to 1980 levels by 2040
• Arctic region recovery by around 2045
• Antarctic region recovery by around 2065
The recovery process continues, but illegal or accidental emissions of banned chemicals could slow progress.
Positive signs prove global cooperation is effective.
15. Role of Individuals in Protecting the Ozone Layer
Simple actions every person can adopt:
• Avoid older appliances that use CFCs
• Choose energy-efficient, eco-friendly products
• Avoid aerosol sprays with harmful propellants
• Ensure proper servicing of cooling equipment
• Support plantation programs and environmental education
• Reduce vehicle pollution by carpooling or using public transport
• Encourage recycling and sustainable consumer behavior
Collective efforts at the household level lead to major environmental benefits.
16. Interesting Scientific Facts About the Ozone Layer
• Ozone absorbs nearly all UV-C rays and a majority of UV-B rays
• Its discovery as a shield for life transformed atmospheric science
• Ozone smells sharp, similar to chlorine, and is toxic at ground level
• Ozone concentration peaks at the tropical upper stratosphere
• Without ozone protection, evolution of life would have been impossible
These facts highlight the unique value of this fragile atmospheric shield.
17. Current Challenges and Future Risk Factors
Despite positive recovery trends, several ongoing issues require attention:
• Illegal production of banned ODS in some industries
• Increase in nitrous oxide emissions from agriculture and vehicles
• Climate change altering stratospheric temperature
• Possible new chemical threats yet to be regulated
• Lack of environmental awareness in developing regions
If not controlled, these factors could delay the recovery of the Ozone Layer by decades.
18. Conclusion
The Ozone Layer is a vital protective shield that allows life to survive and flourish on Earth. The damage caused by Ozone Depletion has shown how deeply human activities affect the planet’s natural systems. However, coordinated actions through global agreements like the Montreal Protocol have proven that when humanity unites, environmental recovery becomes possible.
Continued scientific monitoring, strict regulation of harmful chemicals, adoption of eco-friendly technology, and active public participation are necessary to ensure complete recovery of the Ozone Layer. Protecting this layer means protecting biodiversity, environmental stability, human health, and future generations. The progress made so far is a strong reminder that environmental challenges can be solved when societies take responsibility.